ARLINGTON, Va. – The National Milk Producers Federation today thanked key House and Senate dairy leaders for adding bipartisan momentum to implementing new, greatly needed dairy programs, a top priority for the U.S. Department of Agriculture.
The letters from the House and Senate to Agriculture Secretary Sonny Perdue urge the department to implement dairy-related provisions of the Farm Bill passed in 2018 as swiftly as possible. House Agriculture Committee Chairman Collin Peterson (D-MN) and senior committee member Representative Glenn ‘GT’ Thompson (R-PA) led the House effort, and Senate Agriculture Committee Ranking Member Debbie Stabenow (D-MI) and Senator Roy Blunt (R-MO) led in the Senate. As noted in the letters, the new Dairy Margin Coverage (DMC) program and other improvements in the new farm bill will provide critical help to dairy farmers this year.
The letters, signed by 77 House members and 38 Senators from both parties, also urge active USDA engagement with farmers on multiple levels, including mailings, phone calls and local meetings, as well as collaboration with stakeholders including state officials, cooperatives, producer groups and institutions of higher education.
“We commend Chairman Peterson, Rep. Thompson, Ranking Member Stabenow, Senator Blunt, and their numerous colleagues for drawing attention to the difficulties dairy farmers are enduring,” said Jim Mulhern, president and CEO of the National Milk Producers Federation. “Implementing dairy programs in a fast and farmer-friendly manner is important to NMPF members. We applaud Secretary Perdue for his efforts to commit to a timeline that gives farmers some certainty for financial planning. We need to ensure that outreach is broad and that farm-specific issues that arise during implementation are addressed with flexibility.”
“We look forward to working with USDA to continue to best serve our hard-working, economically stressed producers, and we support the congressional support of this process,” Mulhern said.
Here’s what farmers are saying about implementation efforts:
“Our dairy farmers have endured four consecutive years of low prices – both here in Missouri and across the country. I commend the bipartisan support in Congress for quickly implementing the new dairy provisions of the farm bill, which will be a significant help in times like these. I’m particularly appreciative of my own Senator, Roy Blunt, for co-leading this effort on the Senate side. He has been a friend and ally of dairy dating back to his past service in the House.”
– Randy Mooney, Rogersville, Missouri, dairy farmer. Chairman of the Board for NMPF and Dairy Farmers of America.
“As a constituent dairy farmer of Chairman Peterson’s, I have long appreciated his commitment to standing up for Minnesota’s hardworking dairy farmers. He is a leader on countless issues for dairy and ag, and mostly recently spearheaded efforts to reform dairy policy in the 2018 Farm Bill. The new Dairy Margin Coverage program will make a meaningful difference for all dairy farmers, especially as we head into a fifth consecutive year of low milk prices. I am thrilled the Chairman is leading a bipartisan group of 77 members urging USDA to quickly implement this important program.”
– Steve Schlangen, Albany, Minnesota, dairy farmer. AMPI (Associated Milk Producers Inc.) Chairman of the Board.
“The dairy industry has faced a number of challenges in recent years, ranging from low prices to export challenges to high costs of labor, and Michigan is no exception. I’m extremely grateful for the work that Senator Debbie Stabenow has done on behalf of dairy farmers in Michigan and across the country to improve dairy policy. The improvements made in the farm bill build on helpful reforms made last year, and I’m eager to see them implemented as soon as possible. This bipartisan letter that Senator Stabenow has led helps reinforce this important need.”
– Ken Nobis, St. Johns, Michigan, dairy farmer. NMPF First Vice Chairman.
“Pennsylvania dairy farmers are entering a fifth consecutive year of depressed milk prices. As a dairy farmer myself, I’ve watched countless neighbors of mine close up shop in recent years during this challenging time. Rep. GT Thompson has been an outstanding advocate for Pennsylvania’s dairy farmers through his work on the Agriculture Committee. We look forward to working with him and his colleagues to quickly implement the new Dairy Margin Coverage program, which will provide important benefits to farmers here and around the country.”
– Amanda Condo, Pennsylvania dairy producer. Paul Dotterer & Sons Inc.
###
The National Milk Producers Federation (NMPF), based in Arlington, VA, develops and carries out policies that advance dairy producers and the cooperatives they own. NMPF’s member cooperatives produce the majority of U.S. milk, making NMPF the voice of dairy producers on Capitol Hill and with government agencies. For more, visit www.nmpf.org.
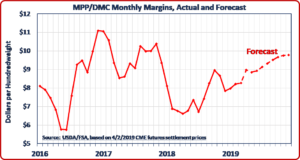 g in MPP during 2018 due to this restriction to enroll retroactively in MPP and collect payments for 2018 for the months during which they were prevented from doing so. Farmers who purchased buy-up coverage under MPP during 2014-2017 are also eligible under the Farm Bill to receive a partial refund of their net payments during those years.
g in MPP during 2018 due to this restriction to enroll retroactively in MPP and collect payments for 2018 for the months during which they were prevented from doing so. Farmers who purchased buy-up coverage under MPP during 2014-2017 are also eligible under the Farm Bill to receive a partial refund of their net payments during those years.

 NMPF is now accepting applications for its National Dairy Leadership Scholarship Program for academic year 2019-2020. The deadline to apply is Friday, April 5.
NMPF is now accepting applications for its National Dairy Leadership Scholarship Program for academic year 2019-2020. The deadline to apply is Friday, April 5.



